Aqueous Extract of Psidium cattleianum as Intracanal Medication: An In Vitro Study of Cell Viability
Christine Men Martins, Thayse Yumi Hosida, Ana Maria Sell, Eloi Dezan Junior and Mirian Marubayashi Hidalgo
DOI10.21767/2469-2980.100043
Christine Men Martins1*, Thayse Yumi Hosida2, Ana Maria Sell3, Eloi Dezan Junior4 and Mirian Marubayashi Hidalgo5
1Dentistry Department, Clinic Integrate Area, Municipal Foundation of Education and Culture FUNEC, Sao Paulo, Brazil
2Restorative Dentistry Department, Pediatric Area, Aracatuba Dental School, Sao Paulo, Brazil
3Basic Health Sciences Department, Immunology Area, Maringa State University, Parana, Brazil
4Restorative Dentistry Department, Endodontic Area, Aracatuba Dental School, Sao Paulo, Brazil
5Dentistry Department, Endodontic Area, Maringa State University, Parana, Brazil
- *Corresponding Author:
- Christine Men Martins
Dentistry Department
Clinic Integrate Area
Municipal Foundation of Education and Culture FUNEC
Santa Fe do Sul, Sao Paulo, Brazil
Tel: +55 (44) 9 9927 8314 E-mail: christinemen@hotmail.com
Received Date: May 04, 2017; Accepted Date: June 17, 2017; Published Date: June 23, 2017
Citation: Martins CM, Hosida TY, Sell AM, et al. Aqueous Extract of Psidium cattleianum as Intracanal Medication: An In Vitro Study of Cell Viability. J Orthod Endod. 2017, 3:9. doi: 10.21767/2469-2980.100043
Abstract
Psidium cattleianum extract has shown activity against oral microorganisms and anti-inflammatory activity, and also tissue biocompatibility. These characteristics aroused interest in its use as an alternative to traditional intracanal medication. The aim of this study was to evaluate the aqueous extract of P. cattleianum cytotoxicity by structural and functional cell viability, in order to use as intracanal medication. The hypothesis is that aqueous extract of P. cattleianum should be used as intracanal medications due to better cytotoxicity response than cell culture medium. Positive control used was RPMI cell culture medium and negative control was water. Cell viability was analyzed after 1 h, 3 h, 6 h, 10 h and 24 h of incubation by exclusion method with trypan blue and MTT assay, using human mononuclear cells (PBMC) and human Periodontal Ligament Cells (PDL) in culture. By trypan blue assay, both PBMC and PDL cells showed an average viability higher (p>0.05) than RPMI when the cells were maintained in aqueous extract. Distilled water showed lowest average viability (p<0.05). By MTT assay, PDL cells showed increasing viability over time when maintained in aqueous extract culture. P. cattleianum was able to maintain the structural and functional cell viability, shoeing a higher performance than positive control. Also, this extract neutralized the detrimental effect of distillated water. The results about biocompatibility assays are promising to indicate the use of aqueous extract of P. cattleianum as an alternative to traditional intracanal medications.
Keywords
Psidium; Cell survival; Materials testing
Introduction
Endodontic treatments are closely associated with Orofacial pain of dental origin, which affect a large part of the population and they are the main representatives of emergency and emergency care, especially in public environments [1,2]. They can also be performed as a result of asymptomatic cases, but in any case, the infection may be present in the root canal system and or the apical region [3]. The suitable chemical-mechanical preparation as well as the use of intracanal medication eliminates infection, prevents recontamination [3-5], and may also prevent or reduce periapical inflammation through biocompatibility [6,7].
Calcium hydroxide is often used as intracanal medication [8,9]. However, new alternatives must be sought, especially considering the worldwide tendency toward the use of medicinal plants, due to advantages such as diversity, flexibility, accessibility, availability and wide acceptance. Furthermore, the World Health Organization reported that medicinal plants would be the biggest and best source of obtaining drugs for humanity [10].
Based on previous studies, Psidium cattleianum shows promise as intracanal medication during endodontic treatment [11- 18]. The aqueous and ethanol extracts of P. cattleianum have shown remarkable antibacterial activity against oral microbiota, anti-inflammatory and potential anticariogenic effects, and biocompatibility [11-15]. However, determining the use and clinical application of this extract requires testing for cytotoxicity [16], sensitization [17], anti-inflammatory and antimicrobial activities [12-15,18], and other issues.
Thus, this study evaluated the aqueous extract of Psidium cattleianum cytotoxicity by structural and functional cell viability, in order to use as intracanal medication. The hypothesis is that aqueous extract of P. cattleianum should be used as intracanal medications due to better cytotoxicity response than cell culture medium.
Material and Methods
The project was approved by the Ethics Committee in Research Involving Humans from the Maringa State University, with the protocol number CAAE: 0108.0.093.000-10.
Extract and controls
The aqueous extract was prepared accordingly to a described protocol [11]. Healthy leaves were selected, washed in tap and deionised water, dried at 37°C for 1 week, ground in a blender until a thin powder was achieved. Aqueous extract was obtained by decoction in deionised water (100 g/600 mL) for 5 min at 100°C and at 55°C for an additional 1 h. The solution was then filter sterilised with 0.22 μm mixed cellulose ester membranes (Millipore™; Billerica, MA, USA). The extract was stored in dark bottles at −20°C until further use.
Culture medium Roswell Park Memorial Institute 1640 (RPMIGibco ®, Life Technologies, USA) was used as a positive control and distilled water served as negative control.
Human mononuclear cells (PBMC)
To obtain the PBMC, 15 ml of venous blood from four donors was obtained aseptically in heparinized tubes with 2 drops of Liquemine® (Roche, Switzerland). The cells were isolated by the method described by Boyum [19] modified. The tubes were centrifuged and the interface between plasma and red blood cells were collected and diluted in sterile Phosphate-Buffered Saline (PBS) and placed over a discontinuous gradient Histopaque® (Sigma Chemical Co., USA). After further centrifugation, the leukocytes were removed via Pasteur pipette and transferred to another tube. Then the washing process was carried out with PBS three times. The cell precipitated was resuspended in RPMI (Life Technologies, USA).
Human periodontal ligament cells maintained in culture (PDL)
The PDL cells were kindly provided by the laboratory of Applied Virology, Santa Catarina Federal University. The cells were kept in an incubator (5% CO2/95% humidity, 37°C) in cell culture bottles (TTP Techno Plastic Products, Switzerland) containing Dulbecco’s MEM (DMEM-Cultilab, Brazil) supplemented with 10% fetal bovine serum (FBS-Cultilab, Brazil) and 1% of a combination of penicillin 10,000 UI/mL, streptomycin 20 mg/ ml and amphotericin B 2 mg/L (PSA-Cultilab, Brazil). The culture medium was changed every 48 or 72 h. After cell confluence the trypsinization was done using 0.25% trypsin solution and Ethylenediaminetetraacetic acid (EDTA-Sigma Chemical Co., USA) for several minutes to release the cells. After neutralization with RPMI (Life Technologies, USA), the cell precipitate was washed, dissolved in a fresh culture medium, and aliquoted in bottles. Each trypsinization yielded a new passage and throughout the experimental period when confluence, cells were replicated to maintain the culture’s optimal viability. The experiments were performed when cells were between the eleventh and twelfth passages, as recommended by ATCC [20] and Oh et al. [21].
Trypan blue assay
The viability was determined by microscopic observation of the cells excluded by staining with trypan blue, a vital stain derived from toluidine. A final concentration of 1 x 106 cells/mL of the two types of cells was incubated in aqueous extract of P. cattleianum or controls during 24 h at 25°C. Samples were collected at 1, 3, 6, 10 and 24 h and analyzed in a Neubauer chamber with an equal volume staining. Cells were considered not viable when impregnated by staining or when degenerated. Three previously trained observers performed the readings under an optical microscope and expressed the results as percentages. They repeated the process four times.
MTT assay
The cells were adjusted to 1 x 104 cells/ml in RPMI (Life Technologies, USA) with 10% FBS (Cultilab, Brazil) and 1% PSA (Cultilab, Brazil), and seeded in 96-well plates (TPP Techno Plastic Products, Switzerland). After incubation (5% CO2 and 95% humidity at 37°C incubator) for 24 h for cell adhesion, the culture medium was discarded, and the extract or control was added. After the incubation times of 1, 3, 6, 10 and 24 h, the experimental medium were removed and MTT solution was added (5 mg/ ml, MTT-thiazole blue, Sigma Chemical Co., USA). The plates stayed in the incubator during 3 h. Then the MTT solution was removed and dimethylsulfoxide (DMSO-Merck, Brazil) was added to dissolve formazan crystals. Cell viability was determined by reading the absorbance of the wells under the wavelength of 550 nm (ASYS Expert plus Microplate Reader, Biochrom, UK). The test was also performed in triplicate with four repeats.
Statistical analysis
Data on the percentage of viable cells were obtained by means of exclusion with trypan blue and PDL to PBMC in culture were collected for the groups in a time-dependent manner. To analyze these results, we used linear mixed effects models with PROC NLMIXED of SAS version 9. The results of the viability of PDL cell culture by MTT method were subjected to ANOVA Factorial through SAS software 9.3.n. The level of statistical significance was set at 5% for all analyses.
Results
Average of cell viability
Table 1 represents the average viability of cells maintained in the aqueous extract and controls. By trypan blue methodology, both PBMC and PDL cells maintained in aqueous extract showed greater viability than in the positive control RPMI, but there is no statistically significant difference (p>0.05). The negative control presented lowest viability (p<0.05). For the MTT, the average Optical Density (OD) was 3.76 times higher in the aqueous extract than the positive control RPMI and 69 times higher than negative control distilled water, but there is no statistically significant difference (p>0.05).
| Trypan Blue (%) PBMC | Trypan Blue (%) PDL | MTT (O.D.)1 PDL | |
|---|---|---|---|
| Extract of Psidium cattleianum | 97.52 | 83.3 | 2.073 |
| RPMI (positive control) | 92 | 78.1 | 0.55 |
| Distilled water (negative control) | 21.5* | 42.4* | 0.03 |
1O.D.: Optical Density. 2Readings performed by three previously trained observers. 3Test was performed in triplicate with four repeats. *Statistically difference between the negative control and other media (p<0.05)
Table 1: Average of cell viability maintained in the aqueous extract and controls.
Cell viability in the aqueous extract of Psidium cattleianum and controls throughout the experimental period
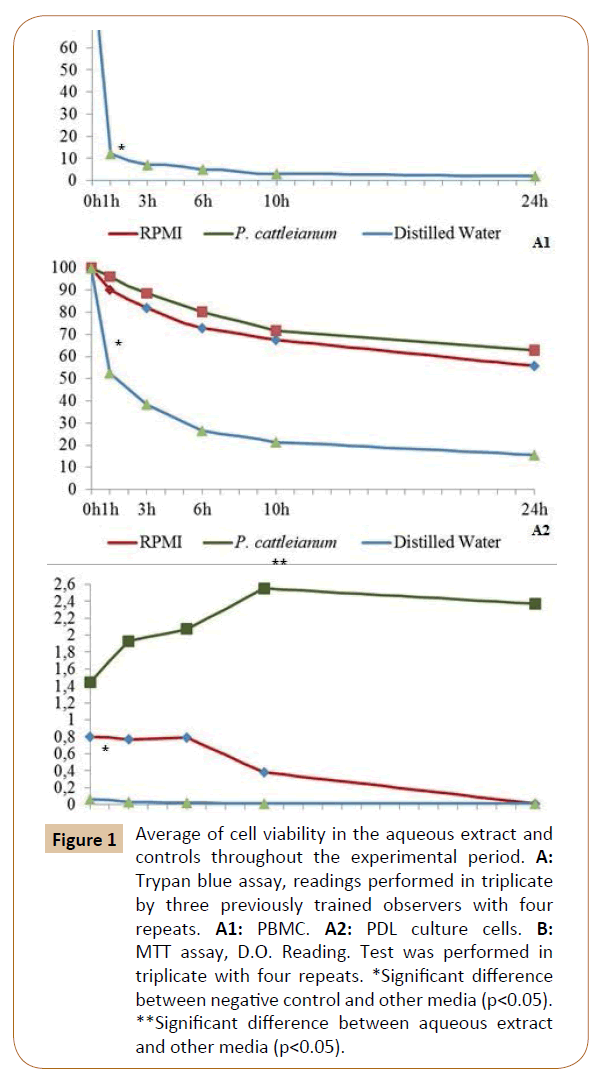
The Figure 1 represents the average cell viability in the aqueous extract and controls throughout the experimental period. Trypan blue assay to PBMC was shown in Figure A1 and to PDL in Figure A2. Aqueous extract maintained structural viability, showing a homogeneous performance during the 24 h, with slightly higher values, but there is no statistically significant difference (p>0.05) than positive control RPMI. The negative control presented lowest viability (p<0.05). In B, the functional viability of PDL maintained in culture and analyzed by MTT is expressed in OD. It demonstrated that aqueous extract allowed higher performance than controls (p>0.05), increasing over time even without statistically significant difference. The diluent control was also the lowest since the first hour (p<0.05).
Figure 1: Average of cell viability in the aqueous extract and controls throughout the experimental period. A: Trypan blue assay, readings performed in triplicate by three previously trained observers with four repeats. A1: PBMC. A2: PDL culture cells. B: MTT assay, D.O. Reading. Test was performed in triplicate with four repeats. *Significant difference between negative control and other media (p<0.05). **Significant difference between aqueous extract and other media (p<0.05).
Discussion
This is the pioneer study of PBMC and PDL cytotoxicity cell viability maintained in the aqueous extract of Psidium cattleianum over 24 h. The aqueous extract was more effective in maintaining cell viability for both cell types, with a homogeneous and slightly higher performance than that of the positive control, and also permitted functional viability of PDL. Thus, regarding to this results, our hypothesis was accepted.
The PBMC was used due to the ease and speed of obtaining it. The PDL was used to approach the in vitro research with the reality, once they are the cells directly involved in the healing after endodontic treatment [22]. Another important factor in cell selection is because these types of cells permit the standardization of cell concentration, in order to detect possible changes.
The trypan blue assay is commonly used in assessing viability and allows the analysis of physical integrity of the cell membrane [19,23]. However, this methodology does not evaluate the real metabolic capacity of the cell. To overcome this gap, we carried out the MTT assay, quantifying the reduction of MTT salt. Through metabolism, MTT salt becomes formazan salt, which is purplish and soluble in DMSO. This makes it possible to quantify cellular metabolic activity via NADPH-dependent oxidoredutases. The reduction of MTT to formazan is directly proportional to mitochondrial activity and cell viability [20,21].
The cells were maintained viable with similar percentages if placed in aqueous extract of P. cattleianum or RPMI. RPMI is usually considered positive control because it is a mixture of salt fortified with amino acids, vitamins and other essential components for cell growth. This medium is a nutrient solution with pH and osmotic concentration ideal for cell culture [24]. The cells maintained in the aqueous extract showed higher viability then those maintained in the RPMI control. Although this is not a statistically significant difference, aqueous extract appears non-cytotoxic, biocompatible, and effective on viability and integrity maintenance.
The aqueous extract was obtained from P. cattleianum plant with distilled water as diluent. Compared to the aqueous extract, the control diluent caused a very high rate of cell death, by the trypan blue assay. Thus, the extract appears to have neutralized the detrimental effect that distillated water performed. The water is a hypotonic medium that can lead to rapid lysis of the cell membrane [25].
When analyzed throughout the experimental period by MTT, the aqueous extract was more efficient than the controls. Aqueous extract showed an increase in OD average, significant (p<0.05) after the tenth hour, which is an unusual finding. The medium usually keeps cell viability constant and its effectiveness to decrease over time. One possible explanation could be the natural antioxidants in the leaf extract of P. cattleianum, as discussed in previous studies [18]. Another possibility is the production and/ or release of mediators or growth factors by the cells themselves after contact with aqueous extract. This increase in OD should be studied in the future, to verify whether it originates with a cell proliferation or increase the metabolic activity of the cells.
In addition to the aqueous extract’s maintenance of the integrity of the membrane and the functional metabolic activity of the cells, it also appears to have favorable biological properties and antibacterial activity, and can influence the demineralization of hydroxyapatite by microorganisms [11-18]. Also, Brighenti et al. [11] showed it may reduce demineralization of the enamel, present acidogenic potential, the viability of microorganisms and the production of extracellular polysaccharide.
All of these properties can be compared with Calcium Hydroxide properties [2-9]. However, Calcium Hydroxide antimicrobial activity is mainly due to alkaline potential [4], while extract presents acidogenic potential [11], so the action path differs between the two substances. Thereby, the aqueous extract of P. cattleianum has potential to be an alternative or to complement intracanal medication. By this way, further studies are needed to analyze others aspects in order to its indication.
Conclusion
So, based on the methodology and results presented, it is concluded that the aqueous extract of Psidium cattleianum was able to maintain structural and functional cell viability, outperform the positive control and neutralize the deleterious effect of distilled water. The results about biocompatibility assays are promising to indicate the use of aqueous extract of P. cattleianum as an alternative to traditional intracanal medications.
References
- Wong NH, Tran C, Pukallus M, Holcombe T, Seow WK (2012) A three-year retrospective study of emergency visits at an oral health clinic in south-east Queensland. Aust Dent J 57: 132-137.
- Shqair AQ, Gomes GB, Oliveira A, Goettems ML, Romano AR, et al. (2012) Dental emergencies in a university pediatric dentistry clinic: a retrospective study. Braz Oral Res 26: 50-56.
- Cotti E, Schirru E, Acquas E, Usai P (2014) An overview on biologic medications and their possible role in apical periodontitis. J Endod 40: 1902-1911.
- Kawashima N, Wadachi R, Suda H, Yeng T, Parashos P (2009) Root canal medicaments. Int Dent J 59: 5-11.
- Mohammadi Z, Abbott PV (2009) Antimicrobial substantivity of root canal irrigants and medicaments: a review. Aust Endod J 35: 131-139.
- Bernades RA, Campelo AA, Junior DS, Pereira LO, Duarte MAH, et al. (2010) Evaluation of the flow rate of 3 endodontic sealers: Sealer 26, AH Plus, and MTA Obtura. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109: e47-e49.
- Holland R, Otoboni Filho JA, de Souza V, Nery MJ, Bernabé PF, et al. (2003) comparison of one versus two appointment endodontic therapy in dogs’ teeth with apical periodontitis. J Endod 29: 121-124.
- Accorinte ML, Holland R, Reis A (2008) Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod 34: 1-6.
- Holland R (1977) Reaction of human periapical tissue to pulp extirpation and immediate root canal filling with calcium hydroxide. J Endod 3: 63-67.
- World Health Organization (WHO) (2002) WHO traditional medicine strategy 2002–2005. Geneva: WHO.
- Brighenti FL, Gaetti-Jardim E, Danelon M, Evangelista GV, Delbem ACB (2012)Effect of Psidium cattleianum leaf extract on enamel demineralisation and dental biofilm composition in situ. Arch of Oral Biol 57: 034-1040.
- Gaetti-Jardim E, Landucci LF, Arafat OKK, Ranieri V, Ramos MMB, et al. (2011) Antimicrobial activity of six plant extracts from the Brazilian savanna on periodontal pathogens. Int J Odontostomatol 5: 249-256.
- Menezes TEC, Delbem ACB, Brighenti FL, Okamoto AC, Gaetti-Jardim E (2010) Protective efficacy of Psidium cattleianum and Myracrodruon urundeuva aqueous extracts against caries development in rats. Pharm Biol 48: 300-305.
- Brighenti FL, Luppens SBI, Delbem ACB, Deng DM, Hoogenkamp MA, et al. (2008) Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 42: 148-154.
- Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, et al. (1999) Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. Ethnopharmacol 67: 203-212.
- Hauman CHJ, Love RM (2003) Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J 36: 75-85.
- Lozoya X, Meckes M, Aboud-Zaid M, Tortoriello J, Nozzolillo C, et al. (1994) Quercetin glycosides in Psidium guajava L. leaves and determination of spasmolytic principle. Arch Med Res 25: 11-15.
- Alves PM, Queiroz LM, Pereira JV, Pereira MS (2009) In vitro antimicrobial, antiadherent and antifungal activity of Brazilian medicinal plants on oral biofilm microorganisms and strains of the genus Candida. Rev Soc Bras Med Trop 42: 222-224.
- Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl 21: 77-89.
- American Type Culture Collection (2001) MTT cell proliferation assay instructions. Catalog Number 30-1010K.
- Oh YH (2005) Cryopreservation of human teeth for future organization of a tooth bank–a preliminary study. Cryobiology 51: 322-329.
- Willershausen I, Wolf T, Kasaj A, Weyer V, Willershausen B, et al. (2013) Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Arch Oral Biol 58: 1232-1237.
- Blömlof L, Otteskog P (1980) Viability of human periodontal ligament cells after storage in milk or saliva. Scand J Dent Res 88: 436-440.
- https://www.cultilab.com.br/paginas/cultivocelular1.3.html
- Marino T, West LA, Liewehr FR, Mailhot JM, Buxton TB, et al. (2000) Determination of periodontal ligament cell viability in long shelf-life milk. J Endod 26: 699-702.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences