Management Of Trauma- And Iatrogenically-Induced Inflammatory Root Resorption: A Case Report
Kian Nikdel,Rebeca Weisleder
| Kian Nikdel, Rebeca Weisleder2* |
| Corresponding Author: Rebeca Weisleder, DDS MEd. Associate professor. Department of Endodontics, UTHealth School of Dentistry at Houston, United States E-mail: beckweis@yahoo.com |
| Received: 27 June 2015 Accepted: 19 August 2015 Published: 28 August 2015 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at Journal of Orthodontics & Endodontics
Abstract
When the pulp becomes necrotic and infected due to the trauma, the bacterial toxins and byproducts can penetrate through dentinal tubules and initiate an inflammatory response in the periodontal ligament causing bone loss, apical periodontitis and external resorption. Disinfection and adequate root filling have been shown to be the treatment of choice when such conditions are present. Different bioceramic materials are been used to achieve a tight seal in the apical portion which may lead to healing.
Background |
||||||||
| When the pulp becomes necrotic and infected due to the trauma, the bacterial toxins and byproducts can penetrate through dentinal tubules and initiate an inflammatory response in the periodontal ligament causing bone loss, apical periodontitis and external root resorption. Disinfection and adequate root filling have been shown to be the treatment of choice when such conditions are present. Different bioceramic materials are been used to achieve a tight seal in the apical portion which may lead to healing. | ||||||||
Report |
||||||||
| This case report describes the use of an alternative approach using mineral trioxide aggregate cement (MTA) and Biodentine for the treatment of maxillary incisors with past history of trauma, root resorption, previous non-surgical endodontic therapy, nonsurgical retreatment and iatrogenic cervical root perforation. At the one-year recall, clinical and radiographic examination shows healing of the periradicular pathosis together with reestablishment of periodontal ligament and lamina dura on the affected teeth as well as healthy periodontal tissues. | ||||||||
Introduction |
||||||||
| Root resorption is a frequent complication of traumatic dental injuries. In the majority of cases pathological root resorption of dental origin is inflammatory in nature. Root resorption develops when the protective superficial layer (internal or external) is damaged or altered as a consequence of trauma associated with the presence of bacteria [1]. It is also a common finding in teeth with apical periodontitis. | ||||||||
| If the pulp becomes necrotic and infected due to trauma, bacterial toxins and degradation byproducts can penetrate through dentinal tubules and initiate an inflammatory response in the periodontal ligament [2]. This can result in root and bone resorption, and formation of granulation tissue which harbors the inflammatory infiltrates including polymorphonuclear leukocytes, lymphocytes, macrophages and plasma cells. Multinucleated giant cells will resorb the root surface while the stimulus persists [3]. | ||||||||
| Mineral trioxide aggregate (MTA) was developed in 1995 and recommended for use in pulp capping, pulpotomies, apical barrier formation in teeth with necrotic pulps and open apices, repair of root perforations, root-end and root canal filling procedures [4]. Biodentine® is a new calcium-silicate-based cement which presents improved characteristics when compared to MTA in regards to difficult handling and long-setting time [5]. | ||||||||
| The aim of this case report is to present an alternative treatment approach using MTA for apexification and Biodentine as root repair material on maxillary incisors with past history of trauma, previous non-surgical endodontic therapy and non-surgical retreatment with concomitant iatrogenic root perforation. | ||||||||
Report |
||||||||
| A 17-year-old female was referred to the Endodontic Clinic at the University of Texas Health Science Center School of Dentistry at Houston for evaluation. The patient presented with pain in the right lateral, right central and left lateral maxillary incisors during mastication. She reported a previous history of trauma to the upper anterior arch in January, 2013. The patient reported that the luxated teeth were repositioned by a general practitioner. A splint was placed for two weeks, after which endodontic therapy was initiated in the three involved incisors. Three months later, non-surgical endodontic retreatment was performed on all of the teeth by the same dentist due to presence of pain and swelling. The patient was on several courses of antibiotics for one year. | ||||||||
| When the patient presented to our endodontic clinic the maxillary right lateral, right central and left lateral incisors revealed moderate sensitivity to percussion and palpation, no response to thermal and electric pulp testing (EPT), mobility grade I (Miller classification) and probing depths ≤3mm. The maxillary canines, left central incisor and mandibular incisors were also tested, and responded positive to cold and EPT tests. No percussion or palpation sensitivity was observed. A sinus tract was located in the mucobuccal fold of the maxillary right central incisor and traced to the apex of that tooth. | ||||||||
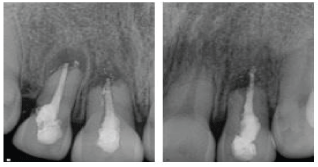
| Radiographic examination showed the presence of periapical radiolucency, root resorption and over-extension but under-filling of root filling material on all teeth. A radiolucency consistent with possible root perforation was observed on the distal aspect of the root of the upper right lateral incisor (Figure 1). | ||||||||
| A diagnosis of previously treated with symptomatic apical periodontitis was attained for maxillary right and left laterals. The diagnosis for the maxillary right central was previously treated with chronic apical abscess (According to the American Association of Endodontics Recommended Diagnostic Terminology). A treatment plan including endodontic retreatments using MTA plugs, nonsurgical and surgical root perforation repair of the maxillary right lateral incisor and permanent restorations was presented. Verbal and written consent for treatment was obtained from the patient and her parent. | ||||||||
| After local anesthesia and rubber dam isolation, tooth #8 was accessed and the existing Thermafil obturation (DENTSPLY Tulsa Dental Specialties DENTSPLY International, Johnson City, TN, USA) was removed. The canal was cleaned and shaped using hand files and 6% sodium hypochlorite (NaOCl) as an irrigant. Calcium hydroxide (Ca(OH)2) paste (Metapaste, META BIOMED Co,. Ltd, Korea) was placed as intracanal medication and the tooth was restored temporarily. The patient returned 4 weeks later for obturation. No symptoms or sinus tract were present at the time. At the following appointment, anesthesia and rubber dam isolation were performed, access was regained and Ca(OH)2 was removed. The tooth was obturated using CollaTape® (Sulzer Calcitec Inc., Carlsbad, California) and MTA cement (Pro-Root MTA; Dentsply-Maillefer, York, Pennsylvania) prepared according to the manufacturer’s instructions and placed with the Micro- Apical Placement (MAP) System. Final obturation was achieved by backfilling the root canal with heated gutta-percha. An orifice barrier (PermaFlo®, Ultradent Products Inc.) was used to seal canal orifices and the access cavity was permanently restored with composite resin. Patient returned after two weeks for retreatment of tooth #10 following the same protocol as for the maxillary right central. | ||||||||
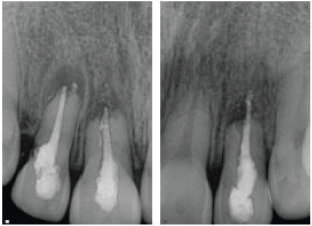
| Two weeks later, the patient returned for retreatment and perforation repair of the maxillary right lateral incisor. After access was gained, a perforation was located in the cervical portion in the distal aspect of the root and repaired internally using CollaTape® and Geristore® Dual Cure Resin Ionomer (Denmat Holdings LLC, Lompoc, California). Retreatment was performed following the same instrumentation and disinfection protocol described above. The root canal space was obturated with MTA. Vitrebond™ Plus Light Cure Glass Ionomer (3M ESPE) was placed as an orifice barrier and the tooth was permanently restored using composite resin (FiltekTM Z250 Universal Restorative, 3M ESPE). Due to the supracrestal location of the perforation, a surgical external repair was also performed. Following anesthesia a full-thickness mucoperiosteal flap was raised, exposing the marginal alveolar bone and the perforation site distal to the tooth. Granulation tissue was removed, the perforation was isolated, dried and sealed with Biodentine®(Septodont). Sutures were placed and removed two days later (Figure 2). | ||||||||
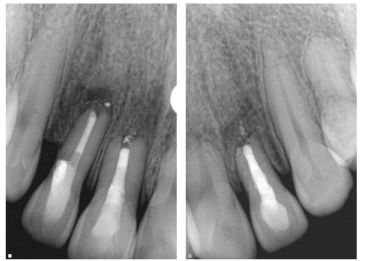
| At the six-month follow up all treated teeth were asymptomatic. All probing depths were less than 3 mm and normal mobility was present in all treated teeth. The maxillary left central incisor was asymptomatic and tested positive to cold and EPT. Radiographically, a decreased radiolucency was observed in all affected teeth (Figure 3). | ||||||||
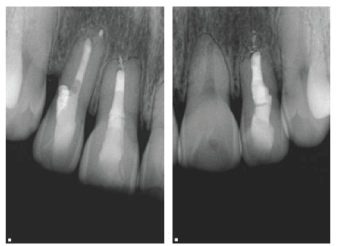
| At the one year follow up, clinical examination revealed no swelling, no percussion or palpation sensitivity, mobility was normal and probing depths were less than 3 mm. Sensibility testing revealed positive response on maxillary left central incisor. Radiographically, healing of periradicular areas, regeneration of new bone and establishment of new lamina dura were observed. The perforation site showed 3 mm periodontal depth measurements and no radiographic evidence of pathology (Figure 4). | ||||||||
Discussion |
||||||||
| In this case report, we present different treatment modalities for teeth with inflammatory root resorption, inadequately sealed root perforation, as well as defective root fillings that contributed to treatment failure. | ||||||||
| Multiple factors may contribute to the persistence and progression of inflammatory root resorption, including luxation injuries to the periodontal apparatus [1,6], root canal infection [7,8], inadequate root canal filling [9-11] and foreign body reaction due to overextended root canal filling into the periradicular tissues [12-14]. Accidental root perforations occur in approximately 2–12% of endodontically treated teeth, with implications for therapy and prognosis [15-19]. Lantz and Persson reported that the most favorable healing response was evident when perforations were sealed immediately [20-22]. | ||||||||
| After appropriate cleaning, shaping and intramedication protocols, MTA was chosen for apical obturation and root canal filling in an attempt to stabilize the affected teeth and promote apexification in conjunction with periradicular healing [4,23-25]. When placed in direct contact with human tissues, MTA releases calcium ions and induces cell attachment and proliferation [26-28]. MTA also allows for an antibacterial environment due to its alkaline pH [29, 30] further stimulating the proliferation of hardtissue producing cells [31,32] and forming a hydroxyapatite-like layer favoring a biologic seal [26,27]. Ozdemir et al. (33) showed that calcium ions diffuse through the dentinal tubules into the resorption bone defect in roots filled with MTA. Further, Martin et al. [34]. concluded that the use of MTA in apexification procedures is effective; moreover it is acceptable to fill the whole root canal with MTA. Based on these observations, MTA has been suggested to promote an ideal environment for healing [4]. | ||||||||
| Fuss and Trope [35] presented a classification of root perforations, based on the time of occurrence, size, and location of the perforation, which will assist the clinician in the choice of the treatment protocol for the best possible results when a perforation is diagnosed. Further, they reported that any biocompatible material with a short setting time and good sealing ability may be useful to seal crestal perforations. In this context, the use of MTA has been described as successful in the repair of supracrestal perforations [36]. Biodentine® is a new tricalcium silicate cement formulation. It has reduced setting time (a few minutes compared with several hours for MTA) and better mechanical properties [37]. Raskin et al. reported on the good sealing ability of Biodentine to resist micro-leakage in cavity margins below the cemento-enamel junction [38]. Guneser et al. [39] reported that Biodentine showed significantly higher push-out bond strength than MTA and a considerably higher performance as a perforation repair material even after being exposed to various endodontic irrigants. Other studies have implied that Biodentine is a bioactive and biocompatible material capable of enhancing human dental pulp stem cells proliferation, migration and adhesion abilities [40, 41]. For these reasons, Biodentine was the material of choice for the external repair of the root perforation of the maxillary right lateral incisor in this case. | ||||||||
| After one year of follow-up, no signs or symptoms of persistent pathologies were present, and complete radiographic healing with visible lamina dura and periodontal ligament space was observed. To our knowledge this is a unique scenario where a case characterized by previous history of trauma, with subsequent non-surgical treatment and retreatment, followed by the occurrence of root resorption and cervical root perforation, achieves healing. The alternative treatment options include periapical surgery, extractions, costly prosthetic appliances or dental implants. Maxillary left central incisor shows root resorption with no apparent periapical radiolucency associated. Cervical root perforation is present on the maxillary right lateral incisor. | ||||||||
Acknowledgments |
||||||||
| The authors thank Dr Ariadne Letra for her valuable editorial contribution to this case report. | ||||||||
Figures at a glance |
||||||||
|
||||||||
References |
||||||||
|
Select your language of interest to view the total content in your interested language
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences